Description
Acellular Corneal Stroma (ACORNEA) FDA Registration No. MDR-04697

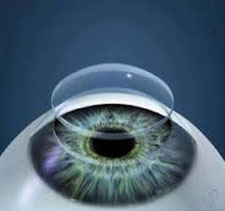
Structural Composition:
Derived from porcine cornea, it is the extracellular matrix (ECM) of porcine cornea prepared through the inactivation of virus and decellularization. The main component of ECM is collagen. This product possesses the collagenous fiber structure of natural cornea, reserving the bowman’s membrane and the partial cornea stroma. Exposed and sterilized under Gamma ray, disposable.
Mechanism:
This product, as the substitute of a cornea, can be used in the treatment of several cornea traumas. It can be grafted to replace the impaired tissue of bowman’s membrane. When it is covered on the lesions, it forms a physical coverage, isolation and protection onto wound bed. Meanwhile, it promotes the corneal epithelial regeneration and matrix synthesis. The product will gradually finish the integration process with the remnant corneal tissues, resulting to the corneal restoration in the aspects of structure and function as normal cornea.
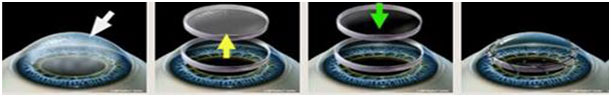
Features of the product
- Good biocompatibility: This product is drawn from the biological cornea. Biocompatible material is superior to the traditional heterogeneous artificial cornea.
- Low immunogenicity: Remove a variety of antigenic material via a special process, main component of it is a proven low-immunogenic substances collagen.
- Excellent optical properties: composition, structure and similar to human cornea. Thetransparency of the cornea is good after implantation.
- Mechanical structural stability: a human cornea similar mechanical properties to meet the needs of clinical surgery.
- Storage and transportation: Does not require preserving fluid. Can be stored and transported at temperature between 2C-10 C. And can be used for corneal transplant in basic hospital
Indication:
For the corneal ulcerations without perforation that pharmaceutical interventions are no use, and the temporary coverage for corneal perforation.
Instruction for Use:
- Preparation before use: The proper debridement is administered; impaired corneal tissues were removed under microscope. Descemet’s membrane, corneal endothelium and minimal posterior corneal stroma are preserved after the debridement. Clean off foreign matters, avoid cauterization to stop bleeding and wash the wound bed with N.S.
- How to use: Open the outer package and withdraw the inner one. Under the aseptic conditions, the inner box is taken out. A proper amount of N.S. is infused into the inner box to rehydrate the product for 10 to 15 minutes. After that, the product is placed with the convexity surface upward onto the wound bed evenly and sutured and fixed. Surgical procedures, medications and postoperative care are the same as the regular lamellar keratoplasty in which the corneal epithelial protective is allowed to be used if necessary.
- Postoperative observation: Closely observe the signs and symptoms of rejection response like aggravated pain, increased secretions, tears, and the edema, dissolution and grow of new vessels around cornea. Corresponding interventions should be carried out in time if necessary.
- After discharge: Pay attention to the protection of affected eye and the prevention of infection.
Contraindications:
- Severe uncontrolled infection on conjunctival sac, lacrimal sac and meibomian gland;
- Moderate or severe dry eye, or Stevens-Johnson syndrome;
- Neutral corneal ulcers;
- Hypophasis
- Allergic to porcine collagen
Precautions:
- ACS should be used immediately after open;
- Any signs of infection are noted after use, routine anti-infective interventions should be carried out;
- Any allergic reactions are noticed during the treatment, the primary surgeon should be informed;
- The use of this product should be directed under the guidance of medical practitioner;
- The use of this product should be strictly followed the indications, for those whose infection are not controlled should be cautious when use;
- Those with immunological corneal ailments should be used with caution;
- Those with uncontrolled glaucoma should be used with caution;
- Follow the routine of medical management when discarded.
Storage and Transportation:
This product should be stored and transported at the temperature between 2°C-10°C
Shelf Life:
6 months





Reviews
There are no reviews yet.